The future of Endoscopy is now....
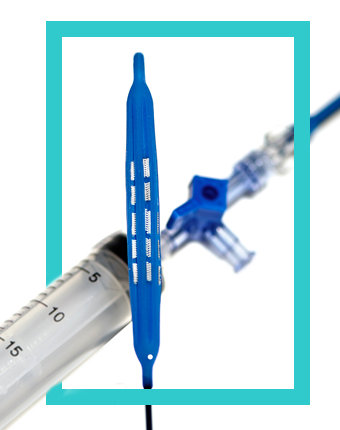
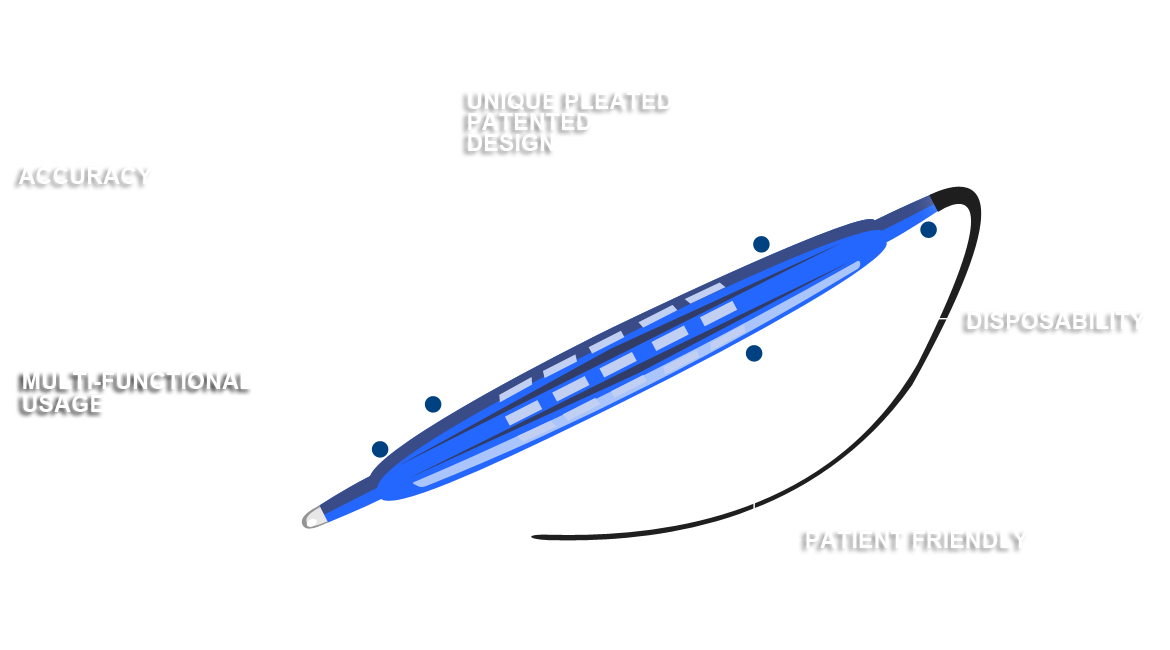
The endoscopy market is about to change. ADN, LLC introduces an innovative balloon system that will revolutionize the way endoscopies are performed. This system facilitates comprehensive sample collection through a patented pleated design that expands to reach the entire circumference of the suspect surface, and then deflates to protect that sample upon retraction. The system is also designed as an agent delivery system for therapeutic, diagnostic or imaging applications. The device is ideal for either a trans-oral or trans-nasal approach, and with patent-pending iterations, no device to date will offer so much flexibility in a self-contained unit.
The ADN system has the additional benefit of disposability. Designed and priced for single use, the ADN system does not require costly, time-consuming, equipment damaging post-procedure sterilization. Practitioners can open a new sterile unit for each new procedure. Two of the world's leading otolaryngologists, Dr. Marshall Strome and Dr. Andrew Blitzer designed the ADN system to address the compromising obstacles that challenge physicians and burden patients. Their unique system streamlines physicians' procedural protocols, saves time and resources, all while improving diagnostic accuracy and patient satisfaction.
Read more
Salient Features –
what makes us unique
What We Offer:

Benefit of Disposability:
The risk and inefficiency of reusable valves is real. 1 out of 2 ready-to-use valves tested are contaminated with bacteria, molds, yeasts and bacterial spores. Reusable valves require a 30-step cleaning procedure that is both difficult and time-consuming. The ADN system offers a 100% disposable iteration where no component is retained for repeated use. Now practitioners can open a new sterile unit for each new procedure as we provide a single-use product; packaged and certified-sterile at delivery, the result is cosmic savings of time and resources.
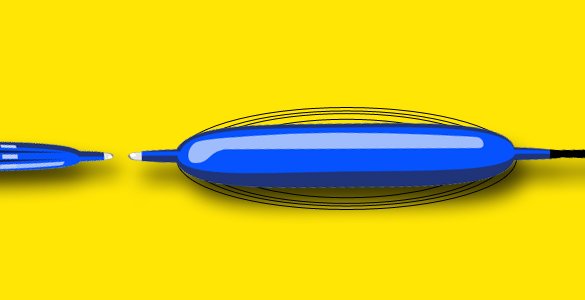
Innovative Balloon System for Comprehensive Sample Collection:
ADN, LLC introduces an innovative balloon system that will revolutionize the way endoscopies are performed. This system facilitates comprehensive sample collection through a patented pleated design that expands to reach the entire circumference of the suspect surface, and then deflates to protect that sample upon retraction. The device is ideal for either a trans-oral or trans-nasal approach, and with patent-pending iterations, no device to date will offer so much flexibility in a self-contained unit.
Inventors of ADN Innovation

Dr. Marshall Strome
(MD, MS, FACS, CEO AND LLC)
Dr. Marshall Strome, board-certified otolaryngologist, is the immediate past Professor and Chairman of the Cleveland Clinic Head and Neck Institute. Dr. Strome has served as president of many professional organizations including The Society of University Otolaryngologists, The American Laryngological Association, The Cartesian Society, The University of Michigan Medical Alumni Association, and The Triological Society (vice president).
Read More

Andrew Blitzer
(MD, DDS, FACS Director Research AND LLC)
Dr. Andrew Blitzer is Senior Attending Otolaryngologist and Director of the New York Center for Voice and Swallowing Disorders. Dr. Blitzer is Professor Emeritus at Columbia University, and past Presidents of both the American Bronchoesophalogical Society and the American Laryngological Association. Dr. Blitzer is a world leader in the management of voice and swallowing disorders and neurolaryngology.
Read More